2 minute read
Pathology is the study of diseases and their effects on living organisms in which tissues, organs, and body fluids are examined to diagnose and manage diseases. Histopathology, or study of sectioned tissues under a microscope, is the gold standard for diagnosis of oncological, infectious, and autoimmune diseases. A domain shift towards Digital Pathology - processing and analysis of high-resolution images of tissue in silico – holds promise to make histopathology assessment faster, more precise and instantly accessible to other medical specialists, improving patient outcomes.
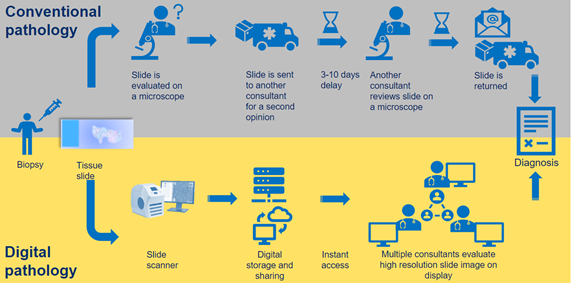
Figure 1 Conventional (upper) and Digital (lower) pathology workflows in comparison.
Pathology contributes to most diagnostic pathways and the rollout of Digital Pathology is expected to improve throughput and accelerate time-to-diagnosis, through faster data availability and analysis.
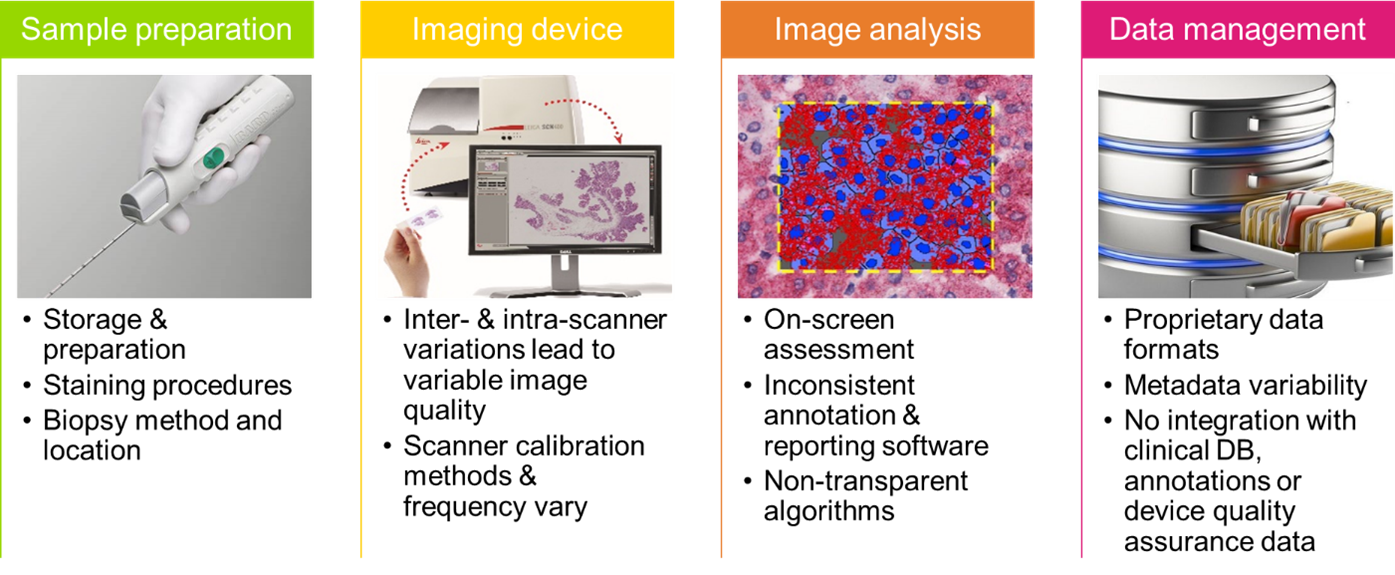
Figure 2 Measurement and standardisation challenges across Digital Pathology workflow stages.
To get a rounded view from the stakeholders and practitioners, we held a workshop that attracted 42 domain experts from industry, academia, healthcare, regulatory authorities, and machine learning and AI developers. The workshop revealed the following challenge areas and issues summarised in a report and published in an NPL-led position paper. The summary of some of those issues are as follows:
Tissue processing: The emerging molecular histopathology techniques are steadily moving towards quantitative evaluation of tissue features (biomarkers). Biomarker quantification is especially challenging due to variations in tissue processing between different instruments, reagents, batches, and laboratories, giving rise to significantly discordant results. Computerised comparable quality assurance procedures are essential to produce comparable tissue samples and reliable quantification.
Whole Slide Imaging (WSI) device calibration: There is no consensus on the scope, methodology and frequency of calibration procedures for WSI devices. Clear calibration guidelines with recommended quality metrics and procedures relying on SI-traceable calibration artifacts would help to achieve consistent data sets that can be used by AI and machine learning algorithms.
Data integration: there is a strong need for a “common language”, i.e., a set of formats and standards, to capture and share WSI data. Linkage mechanisms are required to aggregate data from multiple modalities. The sluggish adoption of a vendor neutral open format such as DICOM hinders data exchange and undermines the key advantage of Digital Pathology.
Use of AI and machine learning algorithms: Although the state-of-the-art AI tools are powerful enough to build predictive pipelines from Digital Pathology images, the best practice to safely deploy these tools in critical areas such as Digital Pathology is not yet defined. Obstacles to the creation of reliable AI systems include access to large well-annotated datasets, large image size, image artefacts, colour variation, regulatory approval, lack of metrics and procedures for training and validation of algorithms, and interpretability/explainability of results. These issues have been highlighted in the context of Deep and Machine Learning in the Data Science publications “Uncertainty evaluation for machine learning” and “Robustness of convolutional neural networks to physiological electrocardiogram noise”.
Molecular imaging: Molecular Pathology involves capturing molecular information (genomic, proteomic, metabolic) to diagnose disease. Molecular information provides insight into tissue composition and function. A range of techniques such as Raman microscopy, mass spectrometry imaging and spatially resolved transcriptomics, can be used to capture
capture and visualise this information for diagnosis. The multitude of techniques and devices calls for comprehensive methods to calibrate different techniques for quantification purposes using reference materials or three-dimensional living samples such as cell organoids. A critical evaluation and guidance on the use of these techniques for specific pathologies is required.
To provide such guidance, we have reviewed the state-of-the-art techniques highlighting the unresolved measurement challenges. The review outlines an effort towards standardisation in molecular pathology imaging and describes the key steps and processes involved in data analysis for Molecular Pathology, highlighting the similarities and differences with Digital Pathology, and where complimentary methods can be used.
NPL’s Digital Pathology team who work on those projects are: Marina Romanchikova, Xavier Loizeau, Dimitrios Tsikritsis, Spencer Thomas, Natalie Belsey, Alex Dexter, Mike Shaw, Michael Adeogun and Susan Rhodes
Keep updated with NPL’s news for further insight into this area of research
01 Mar 2023